The WHO Confirms that the Covid-19 PCR Test is Flawed: Estimates of “Positive Cases” are Meaningless. The Lockdown Has No Scientific Basis

All Global Research articles can be read in 27 languages by activating the “Translate Website” drop down menu on the top banner of our home page (Desktop version).
***
The Real Time Polymerase Chain Reaction (RT-PCR) test was adopted by the WHO on January 23, 2020 as a means to detecting the SARS-COV-2 virus, following the recommendations of a Virology research group (based at Charité University Hospital, Berlin), supported by the Bill and Melinda Gates Foundation. (For Further details see the Drosten Study)
Exactly one year later on January 20th, 2021, the WHO retracts. They don’t say “We Made a Mistake”. The retraction is carefully formulated.
While the WHO does not deny the validity of their misleading January 2020 guidelines, they nonetheless recommend “Re-testing” (which everybody knows is an impossibility).
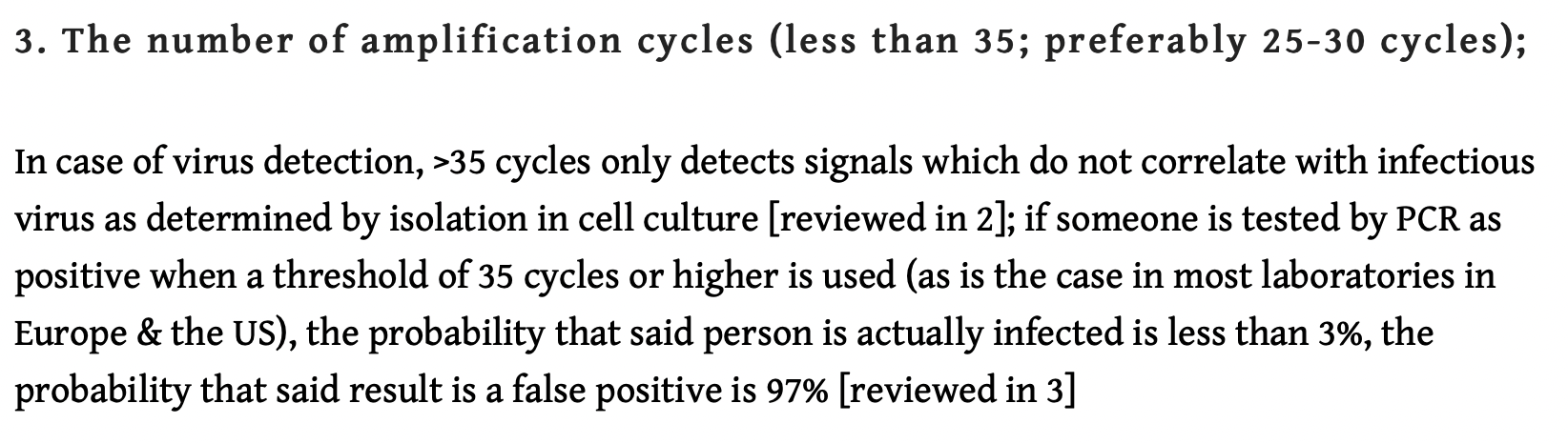
The contentious issue pertains to the number of amplification threshold cycles (Ct). According to Pieter Borger, et al
The number of amplification cycles [should be] less than 35; preferably 25-30 cycles. In case of virus detection, >35 cycles only detects signals which do not correlate with infectious virus as determined by isolation in cell culture…(Critique of Drosten Study)
The World Health Organization (WHO) tacitly admits one year later that ALL PCR tests conducted at a 35 cycle amplification threshold (Ct) or higher are INVALID. But that is what they recommended in January 2020, in consultation with the virology team at Charité Hospital in Berlin.
If the test is conducted at a 35 Ct threshold or above (which was recommended by the WHO), the virus cannot be detected, which means that ALL the so-called confirmed “positive cases” tabulated in the course of the last 14 months are invalid.
Below is the WHO’s carefully formulated “retraction”. The full text with link to the original document is in annex:
WHO guidance Diagnostic testing for SARS-CoV-2 states that careful interpretation of weak positive results is needed (1). The cycle threshold (Ct)needed to detect virus is inversely proportional to the patient’s viral load. Where test results do not correspond with the clinical presentation, a new specimen should be taken and retested using the same or different NAT technology. (emphasis added)
WHO reminds IVD users that disease prevalence alters the predictive value of test results; as disease prevalence decreases, the risk of false positive increases (2). This means that the probability that a person who has a positive result (SARS-CoV-2 detected) is truly infected with SARS-CoV-2 decreases as prevalence decreases, irrespective of the claimed specificity.
“Invalid Positives”
This is not an issue of “Weak Positives” and “Risk of False Positive Increases”.
What this admission of the WHO confirms is that the estimate of covid positive from a PCR test (with an amplification cycles of 35 cycles or higher) is invalid. In which case, the WHO recommends retesting: “a new specimen should be taken and retested…”.
That recommendation is pro-forma. It won’t happen. Millions of people Worldwide have already been tested, starting in early February 2020. Nonetheless, we must conclude that unless retested, those estimates (according to the WHO) are flawed. I should mention that there several other flaws regarding the PCR test which are not addressed in this article. (See Michel Chossudovsky E-book, Chapter II)
From the outset, the PCR test has routinely been applied at a Ct amplification threshold of 35 or higher, following the January 2020 recommendations of the WHO. What this means is that the PCR methodology as applied Worldwide has in the course of the last 12-14 months led to the compilation of faulty and misleading Covid statistics.
And these are the statistics which are used to measure the progression of the so-called “pandemic”. Above an amplification cycle of 35 or higher, the test will not detect the virus. Therefore, the numbers are meaningless.
It follows that there is no scientific basis for confirming the existence of a pandemic.
Which in turn means that the lockdown / economic measures which have resulted in social panic, mass poverty and unemployment (allegedly to curtail the spread of the virus) have no justification whatsoever.
According to scientific opinion:
“if someone is tested by PCR as positive when a threshold of 35 cycles or higher is used (as is the case in most laboratories in Europe & the US), the probability that said person is actually infected is less than 3%, the probability that said result is a false positive is 97% (Pieter Borger, Bobby Rajesh Malhotra, Michael Yeadon, Clare Craig, Kevin McKernan, et al, Critique of Drosten Study)
At the time of writing (March 2021), despite the WHO retraction, the test is being used extensively to hike up the numbers with a view to sustaining the fear campaign, justifying the ongoing lockdown policies as well as the implementation of the Covid vaccine.
The WHO confirms that the Covid PCR test procedure as applied is invalid. It’s a “pack of lies”.
There is absolutely no scientific basis for implementing the Covid Vaccine.
Both the WHO and the scientific assessment of Pieter Borger, et al (quoted above) confirm unequivocally that the tests adopted by governments to justify the lockdown and the destabilization of national economies are INVALID.
Moreover, those PCR tests are not routinely accompanied by a medical diagnosis of the patients who are being tested.
***
See:
Michel Chossudovsky‘s E Book entitled: (Ten Chapters)
Full text of the WHO directive dated January 13, 2021
Annex
Nucleic Acid Testing (NAT) Technologies that Use Polymerase Chain Reaction (PCR) for Detection of SARS-CoV-2
Product type: Nucleic acid testing (NAT) technologies that use polymerase chain reaction (PCR) for detection of SARS-CoV-2
Date: 13 January 2021
WHO-identifier: 2020/5, version 2
Target audience: laboratory professionals and users of IVDs.
Purpose of this notice: clarify information previously provided by WHO. This notice supersedes WHO Information Notice for In Vitro Diagnostic Medical Device (IVD) Users 2020/05 version 1, issued 14 December 2020.
Description of the problem: WHO requests users to follow the instructions for use (IFU) when interpreting results for specimens tested using PCR methodology.
Users of IVDs must read and follow the IFU carefully to determine if manual adjustment of the PCR positivity threshold is recommended by the manufacturer.
WHO guidance Diagnostic testing for SARS-CoV-2 states that careful interpretation of weak positive results is needed (1). The cycle threshold (Ct) needed to detect virus is inversely proportional to the patient’s viral load. Where test results do not correspond with the clinical presentation, a new specimen should be taken and retested using the same or different NAT technology.
WHO reminds IVD users that disease prevalence alters the predictive value of test results; as disease prevalence decreases, the risk of false positive increases (2). This means that the probability that a person who has a positive result (SARS-CoV-2 detected) is truly infected with SARS-CoV-2 decreases as prevalence decreases, irrespective of the claimed specificity.
Most PCR assays are indicated as an aid for diagnosis, therefore, health care providers must consider any result in combination with timing of sampling, specimen type, assay specifics, clinical observations, patient history, confirmed status of any contacts, and epidemiological information.
Actions to be taken by IVD users:
- Please read carefully the IFU in its entirety.
- Contact your local representative if there is any aspect of the IFU that is unclear to you.
- Check the IFU for each incoming consignment to detect any changes to the IFU.
- Provide the Ct value in the report to the requesting health care provider.
Notes
1. Diagnostic testing for SARS-CoV-2. Geneva: World Health Organization; 2020, WHO reference number WHO/2019-nCoV/laboratory/2020.6.
2. Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ. 1994 Jul 9;309(6947):102. doi: 10.1136/bmj.309.6947.102.
*
Note to readers: please click the share buttons above or below. Forward this article to your email lists. Crosspost on your blog site, internet forums. etc.








Geen opmerkingen:
Een reactie posten